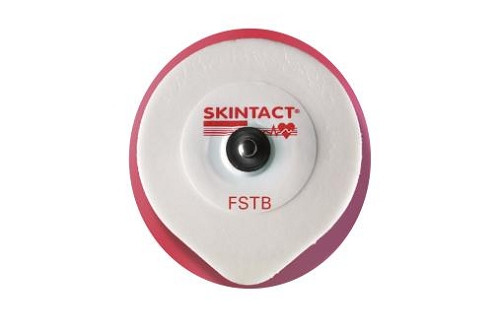
Skintact-FS-TB ECG Gel Electrode
Standard Ground Shipping is now $24.99 up to $400. FREE SHIPPING on orders $400+ in Continental USA ONLY. Restrictions Apply. Oversize, Heavy and Truck Deliveries are Exempt.
Always the correct part
We know our products
Post Sales Support
Free shipping over $350

Manufactured using Leonhard Langs unique C-LINE technology which ensures that every electrode will be fresh providing you with exceptional consistency in quality of recordings at very affordable prices. Skintact electrodes are made from environmentally friendly materials, they can be disposed of using standard waste disposal procedures unless your clinical protocol or local code requires special treatment.